Increased options for the care of complex CV patients requires a renewed, collaborative & evidence-based approach to care
In today’s management of patients with acute myocardial infarction with cardiogenic shock, high-risk percutaneous coronary intervention (PCI), heart failure and valvular heart disease, the use of advanced technologies is on the rise—with growth rates only expected to increase. As indications are expanded, and usage of these therapies increases, it’s more important than ever to provide patient-centered recommendations using a heart team approach.
Patient-centered recommendations take into consideration the patient’s wishes, the risk of the various options, the need for additional procedures and quality of life. Utilization of the heart team approach to care is a Class I indication from both the American College of Cardiology (ACC) and the Society of Thoracic Surgeons (STS). The heart team also plays a critical role in monitoring quality on an ongoing basis, defining treatment protocols and standardizing care.
Expanded indications for CV devices will require guidance
TAVR (transcatheter aortic valve replacement) was originally indicated for high-risk patients only. Later, it received approval from the Food and Drug Administration (FDA) for intermediate-risk patients, and in August of 2019, it received FDA approval to be utilized in low-risk patients.

“In simple terms, it means that this procedure is about to grow exponentially across the marketplace,” says Robin Cunningham, MSN, RN, former HealthTrust Clinical Research Director. “The approved use in low-risk patients paves the way for an even more rapid expansion, as experts predict the TAVR procedure will begin replacing a large portion of surgical valve replacement volume in the next couple of years.”
Similarly, TMVR (transcatheter mitral valve repair) has recently been approved for expanded indications. The only FDA-approved TMVR device on the market, the MitraClip, was originally approved to treat patients with degenerative mitral valve regurgitation. In March 2019, the ACC presented the COAPT clinical trial, which demonstrated that patients with functional mitral regurgitation had a decrease in hospitalization and an improved quality of life. Subsequently, the FDA approved the device for functional mitral regurgitation as well.
“For the MitraClip to get this kind of indication from the FDA is significant,” Cunningham explains, “since aortic valve and mitral valve diseases are the two predominant valve disorders across the world.”
A heart team approach to these two devices will help ensure that this “explosive growth” is guided by evidence and collaboration. Cunningham says, “One of the Centers for Medicare & Medicaid Services (CMS) requirements for TAVR and TMVR is that there be a heart team approach. The facility that makes the decision to perform these procedures needs to have in place, and participating on every case, a heart surgeon, interventional cardiologist, clinicians and specific equipment.” The CMS also requires the collection and recording of data to monitor outcomes.
This mindset is at the heart of making sure these devices are used properly to improve healthcare, Cunningham says.“At the end of the day, you want these patients to recover in a way that is successful.”
Increased use of Impella highlights the need for a heart team
The Impella CP (Abiomed) is approved to provide support in patients with an acute myocardial infarction complicated by cardiogenic shock (AMI-CS). Designed for short-term support, these devices are intended as a bridge for myocardial recovery, or as a bridge to further therapies such as an implantable heart assist device or cardiac transplantation. Another cardiac assist device, the Impella RP (Abiomed), is approved for emergency, temporary support of the right ventricle.
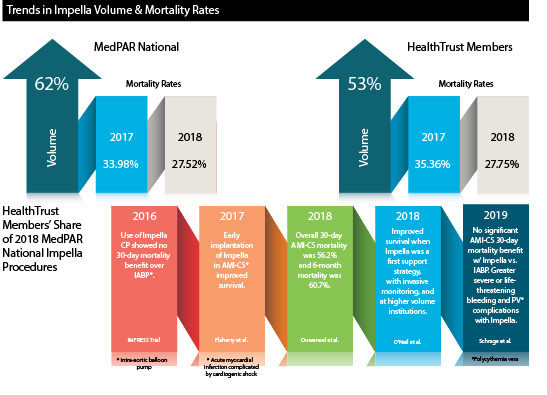
Historically, in-hospital mortality for AMI-CS has been about 30%, based on data from the ACC Cath/PCI registry. Recent evidence and clinical trials (see graphic below) have shown mixed results when evaluating the impact of Impella use on mortality rates, compared to other mechanical support devices and conservative medical therapy.
In fact, among HealthTrust members, between 2017 and 2018, the use of Impella devices for Medicare beneficiaries increased by 53%, while mortality rates during the same period remained near 30% (27.75%).

Impella therapy requires a more collaborative approach to decision-making due to the complexity and severity of patients with AMI-CS, according to HealthTrust AVP of Clinical Data Solutions Kimberly Wright, RN. “Heart teams should monitor real-world outcomes for their patients with AMI-CS to guide appropriate patient selection and application of best practices for these high-risk and resource-intensive patients,” she says.
In May of this year, the FDA issued a warning to cardiologists, cardiothoracic surgeons and transplant surgeons that the post-approval data for Impella RP indicated a lower survival rate compared to premarket survival rates. The FDA approved revised labeling for the Impella RP System to better identify the sub-group of patients most likely to benefit from the device.
“Given the complexity of patients and expansion of advanced therapies, analysis of care quality, appropriateness and patient outcomes has never been more important,” Wright says. “The HealthTrust team is uniquely poised to assist hospitals with this goal.”
For more information on HealthTrust care redesign and on-site assessments, contact Kimberly Wright at kimberly.wright@healthtrustpg.com